Chemotherapy principles
Episode Notes
Cell cycle and cell growth in tumors
Normal tissues are either static, expanding, or renewing
Static tissues are the safest from cytotoxic therapies because they’re rarely dividing (i..e nerves, muscles)
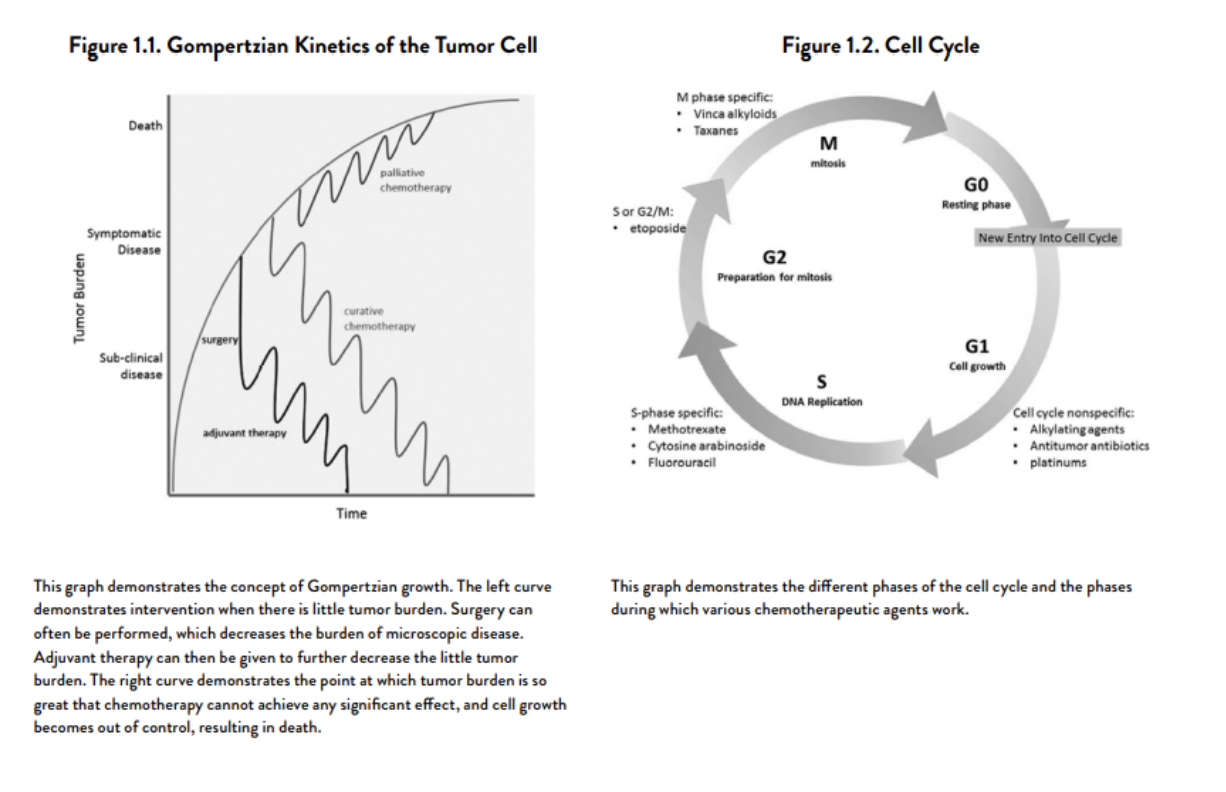
Gompertzian growth kinetics: they have really fast, exponential growth early on, and as they get bigger the growth of individual cells slows and plateaus, however since the tumor size is overall much larger, when they double in size the effect is dramatic. So even though proliferation decreases and doubling time increases, doubling in size happens much more quickly in larger tumors.
Cell cycle
Cells that are in the G0 phase are relatively safe from chemotherapy
Combine chemotherapeutic drugs with different properties (cell cycle specific + cell cycle nonspecific) to optimize effect
“Curability” is inversely proportional to the number of tumor cells in active cell cycles
Tumor kinetics
Log Kill: Only a fraction of cells killed w/ each treatment, and not a constant number. Only when logarithmic kill is very large (>99%), and treatment is repetitive, can single agent chemotherapy be curative
Goldie-Colman hypothesis: mutation toward resistance occurs spontaneously at a rate of one mutation per 1 million cell divisions or higher (with tumor environment or pressures placed on the tumor environment such as chemo); thus, the likelihood of spontaneous mutation increases as the mass increases; drug exposure further increases mutations
Pharmacokinetics
Compartment Models
1 compartment model: drug remains in IV space after IV injection (not true for most drugs)
2 or 3 compartment model (how we understand pharmacokinetics for most of the drugs we use): ultimate targets are beyond the IV space and are in tumor tissues. Takes into account total volume and space of tumor tissues (extravascular space and extravascular, extracellular space)
AUC
“Area under the plasma disappearance curve”
Determined by calculating the concentration x time
Calvert formula is used to calculate AUC: (target AUC)*(GFR+25)
Use the Cockcroft-Gault equation to determine creatinine clearance/eGFR: [(140-age)(wt)(0.85)]/[(72)(Cr)]
Minimum Cr used for the equation should be 0.7
How we dose carboplatin and other drugs
Bioavailability
extent and rate at which the drug becomes completely available at the intended target site
impacted by mode of administration, properties of the chemotherapeutic agent (molecular weight, whether it needs to be activated from prodrug form within the body, methods of elimination), as well as properties of the target organ (membrane permeability)
Dose density vs dose intensity
Dose-dense chemotherapy refers to a chemotherapy treatment plan in which the same total dose of chemotherapy is given over a shorter interval than a standard regimen. In other words, the interval between successive treatments is reduced.
Dose intensity, on the other hand, is the amount of drug delivered per unit of time, expressed as mg/m2/week, regardless of the schedule or route of administration.
Current recommended dosages for each cancer are an attempt to optimize dose intensity to achieve maximal oncologic benefit while not creating overwhelming side effects/toxicity.
RECIST v 1.1 Criteria
uses imaging, most commonly CT, to assess disease status
“Target” lesions
Up to 5 total and 2 per organ can be selected
Tumor lesions must be larger than 10 mm by CT scan in the long axis or 20 mm by chest X-ray
Lymph nodes w/ a short axis of >= 15 mm are considered measurable and assessable
Response definitions
Complete response (CR) is the disappearance of all target lesions
Partial response is a >= 30% decrease in the sum of diameters of target lesions, taking the baseline sum diameters as reference
Progressive disease is >20% increase in the sum of diameters of target lesions, taking as reference the smallest sum that target lesions have been at any point in treatment. Sum of diameters must also have an absolute increase of at least 5 mm.
Stable disease is the catch-all category if doesn’t meet the above criteria
Figure 1.
Chemotherapy distribution compartment model, from the SGO ConnectED fellows bootcamp lecture (reference 9 on main references page)
Figure 2.
Tumor growth kinetics (From Antineoplastic Therapies for Gyn Malignancies, reference 5 on main reference page)